![[image of flower]](../ima/flowXS_06.gif)
![[image of flower]](../ima/flowXS_06.gif)
Glossary D
- Distillation
A process of separating a desired liquid from a mixture by heating the mixture to
vaporize the
desired liquid, and then condensing it into another receptacle. By contrast, in
evaporation the liquid is vaporized, but not
condensed. The mixture might be two or more liquids with different boiling points,
or a mixture of liquids and solids. Distillation is an ancient process and was always
widely used in laboratories and industrial scale chemical processing. Distillation is
used to produce gasoline, alcoholic
beverages, chemicals, and drinking water. It also is used to separate chemical
compounds in laboratories. In the photo
below, an example of straight distillation using a Liebig condenser, a long tube surrounded by a
water jacket through which cold water is continuously pumped, impure water in one flask is
heated to
produce a vapor, which is then cooled by passing it in a separate tube next to cold water and
then condensed to pure water in another flask. The impurities with a lower boiling
point do not boil and are left behind.
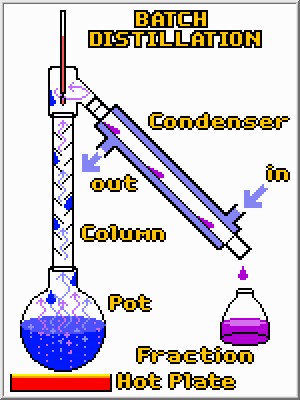
Straight distillation is used to separate a liquid from a solid or 2 liquids that have very
different boiling points, e.g., drinking water from sea water.
If the boiling point of 2
liquids are close, then fractional distillation is used. The still head is separated from the
still pot by a column in which the temperature is lower at higher heights. As the vapor
rises in the column, it condenses and descends. Thus, the descending liquid is always in
contact with the rising vapor, which becomes richer in the more volatile substances.
Likewise, the condensing liquid becomes richer in less volatile substances. Eventually,
the desired volatile substances are collected at the head and distilled in a liquid.
Fractional distillation is used to separate crude oil into various components, such as kerosene,
gasoline, and lubricants, a process called
petroleum refining. This type of distillation is also
used to separate oxygen, nitrogen
and other gases from liquid air.
Steam distillation is used to separate organice compounds with high vapor pressures that are
immiscible in water. They might decompose or oxidize at their own boiling points.
Steam is passed into the organic and water layers to cause the mixture to boil and
distill according to their vapor pressures. The purified organic layer is then separated.
Aniline is separated this way.
In destructive
distillation, a faw substance is decomposed and the materials it released are separated by
distillation. Coal is broken down into coal gas,
benzene, toluene, and other hydrocarbons by this method.
Vacuum distillation is distillation under reduced pressure. It is used to boil large
molecular compoounds at lower temperatures, so that they will not decompose, which they
would at high temperatures. It is used to produce highly viscoua lubricating oils from
crude oil residue, called bitumen (asphalt), used for road surfaces. This method also is
used to remove unpleasant odors from oils, such as cottonseed and soybean oils, to make
them more desirable as cooking oils.
How 726,727
- Ductility
A measure of plastic deformation without fracture. It is an important
property of metals because it indicates the ability to elongate and indent, such as in
drawing wire and stamping sheet metal.
- Dust Bowl
The Great Dust Bowl is a southern
Great Plains region included about 96 million acres
that in the 1930s experienced severe lost of topsoil and farm land. This region
included parts of Kansas, Oklahoma, Texas, New Mexico, and Colorado. Severe
droughts, coupled with poor soil management and high winds, literally blew the
valuable
topsoil away. The U.S. Department of
Agriculture established the Soil
Conservation Service in 1935 to deal with the problem. Considerable improvement
was made in the soil management of water and wind erosion of that region since the
mid-1950s. The remedies
include planting trees and grasses to reduce soil runoff and providing windbreaks and
irrigation systems to maintain adequate water content.
Considine 2607
- Dye
A colored substance that has an affinity for the substrate material to which it is being applied.
It is used to color clothing and hair, detect porosity and cracks in metals, and
microscopic organisms for study.
A dye is usually soluble and used in an aqueous solution that may require a
mordant to improve its fastness on a fiber.
In contrast, a pigment generally has no affinity for
the substrate, and is insoluble. Dye use is ancient, used by all known cultures for at
least 5,000 years.
Wiki n.p.
They were used in Egypt by 3,000 BCE and many colors were
developed by 1,400 BCE. Natural dyes were obtained from animals, vegetables and
minerals with little processing. Around 1,200 BCE, Tyrian purple was invented.
It resisted fading from the sun and wear and was highly prized and expensive. Most
dyes came from the plant kingdom, such as
roots, berries, bark, leaves and wood. Some examples of natural dyes are
tyrian purple that came from a snail
in the harbor of Tyre, cochineal from an insect, indigo, madder
and alizarin
from plants and greens and yellow from copper sulfate. In 1856, a British chemistry
student, William Perkin, accidently
invented the first synthetic dye, eventually called mauve, while experimenting with
coal tar in search of a synthetic quinine.
This invention led him and
other chemists to discover many other synthetic dyes and promoted research
into medicines.
Asimov 372
|








![[image of flower]](../ima/flowXS_06.gif)
![[image of flower]](../ima/flowXS_06.gif)



